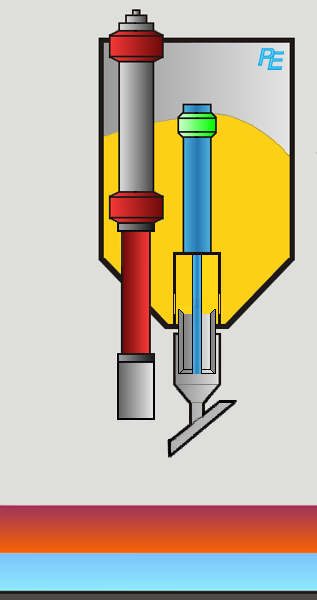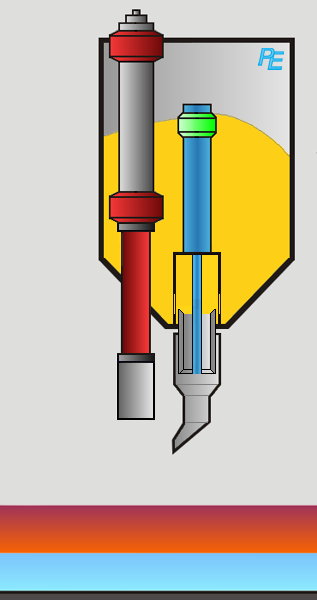


Alumina Hopper
Crust Breaker
Alumina Feeder
Anode Cover
Top Crust
Cryolite Electrolyte
Aluminum Metal Pad
Please hit a button
Crust
Breaker

Pechiney
Feeder

Alcoa
Feeder
Feeding Parameter
The Hall - Heroult Process
The conventional Hall - Héroult process produduces aluminum by high temperature smelting. Alumina (an oxide of aluminum Al203) is dissolved in molten cryolite (Na3AlF6). The electrolytic process decomposes the oxide between carbon and aluminum electrodes at about 950 °C to aluminum and oxygen. The electrolytic process consumes alumina i.e alumina must be added to the elctrolyte to keep its concentration constant.
Alumina Feeding
Two devices execute the aluminum feeding task: first the crust breaker opens a hole in the top crust. The top crust is a layer of solidified electrolyte on top of the electrolyte. Then the alumina feeder drops a defined amount of alumina into the elctrolyte. The process computer controls the actions of both devices.
Visualization of the Feeding Process
You may activate the crust braker or alumina feeder by clicking on the corresponding button or on the device itself.
You find more information how to use this web page on the corresponding User's Guide webpages. The Theory web pages discuss the theoretical background of alumina feeding.
close
09Okt16 15H00